Lele Kelechi Charity, Verla Andrew Wirnkor, Amaobi Collins Emeka, Ajero Adaugo Isioma, Enyoh Christian Ebere and Verla Evelyn Ngozi
Water is highly essential for both industrial and human activities, and so scarcity of good drinking water in both rural and urban areas in developing countries is of great concern. Six borehole water samples obtained from twelve different villages in Uzoubi, Umuna, Orlu labeled A-F where collected and investigated for physicochemical characteristics and heavy metals. Result showed that all metals except for Cadmium (Cd)(0.0492 mg/L), Nickel (Ni) (0.032 mg/L) and Mercury (Hg)(0.0018 mg/L) had concentration higher than WHO permissible limit for safe drinking water. All metals exhibited low to moderate contamination factor except for Ni (6.0) which showed high contamination factor at borehole B, Cd at borehole A (3.6), B (4.2), D (40) and Hg (3.0) at borehole A. Individual pollution load index for all metals at the different sampled borehole water were low, indicating that the water is unpolluted. Ranking base on decreasing order of mean anthropogenic input, followed the order Cd (930%)>Ni (139.2%)>Hg (125%)>Cu (59.33%)>Zn (54.43%)>Se (50.8%)>Ag (49.14%)>Ar (33.33%)>Cr (30.0%)>Pb (24.33%). HQ via ingestion for both adult and children follow the decreasing order respectively Cr>Pb>Cu>Zn>Cd>Ni; Ni>Cr>Pb>Cu>Zn>Cd. HQ via dermal contact for both adult and children follow the decreasing order respectively Cu>Zn>Pb>Cd>Cr>Ni; Cr>Cd>Zn>Cu>Pb>Ni. HI via ingestion and dermal contact for children (8.47E-04, 1.33E-03) showed that children were more exposed to health dangers when compared to adult for prolong consumption of the borehole water. WQI of the five sampled borehole water were categorized under excellent water quality and follow. Ranking the WQI follows the order; E>A>D>F>B=C respectively. A positive correlation was observed for the borehole water samples confirming anthropogenic input. Though not polluted the investigated boreholes water was found to be contaminated and requires proper treatment before consumption as prolong usage could lead to serious health risk arising from metals bioaccumulation in human body.
Emmanuel Amuntse Yerima, Raymond Bwano Donatus, Ifeoma Juliet Opara, Godwin Ogbaji Egah and Joseph Dennis Ani
Increasing industrial activity around the world has left behind large number of contaminants such as heavy metals which can easily get into food chain and bio-cumulate in tissues of living organism with detrimental effect. This study was carried out to assess the impact of activities in a sack production and packaging company on the level of heavy metal on soils around the industrial layout. The results obtained from the soil analysis reveals that the pH, organic carbon and organic matter content of the test and control soils were (8.40 ± 0.20 and 8.51 ± 0.01), (1.76 ± 0.030 and 0.92 ± 0.02%) and (3.03 ± 0.33 and 0.55 ± 0.05%) respectively. While the available phosphorus content, nitrogen and effective cation exchange capacity of the test and control soil samples were (3.62 ± 0.02 and 4.11 ± 0.10%), (0.251 ± 0.01 and 0.078 ± 0.001%) and (65.59 ± 0.05 and 14.78 ± 0.01 Meq/100 g) respectively which were within the limits of normal agronomical soil. The mean ± standard deviation of heavy metal concentrations in the test and control soil were Fe (4625.32 ± 0.252 mg/kg and 3676.44 ± 0.57 mg/kg), Ni (48.20 ± 0.128 mg/kg and 27.50 ± 0.11 mg/kg) Co (36.85 ± 0.046 mg/kg and 37.05 ± 0.044 mg/kg) and Pb (321.45 ± 0.038 mg/kg and 174.23 ± 0.088 mg/kg) respectively where the mean concentrations of the heavy metals in the soil are in the order: Fe>Pb>Ni>Co while Cd and Cr were below detection limit. The impact of the industrial activities determines using indices such as Geo-accumulation index and contamination factor indicates high contamination of the soil with lead, a non-essential element responsible for lead poisoning. Also, the statistical analysis showed a general significant difference between the mean content of heavy metal between the soil around the industry and the soil around the control site suggesting that metal enrichment is likely due to anthropogenic activity since the metal level in the test soil were generally higher with the exception of cobalt.
Soumyadeep Mukhopadhyay, Sumona Mukherjee, Mohd Ali Hashim, Sahu JN, Nadia Martinez Villegas and Bhaskar Sen Gupta
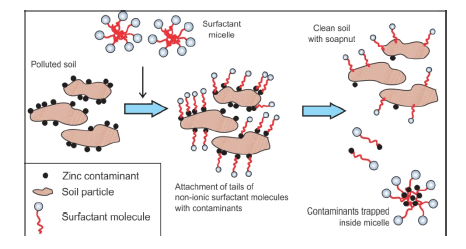
This study explores the possible application of a biodegradable plant-based surfactant saponin obtained from Sapindus mukorossi or soapnut, for washing zinc from contaminated soil. Batch experiments were conducted by varying pH, surfactant concentration and soil: solution ratio and compared to SDS, a synthetic surfactant. It was observed that soapnut was more efficient than SDS due to its lower pH. Also, the surfactants were more effective at higher concentrations. Soapnut solution removed more than 73% zinc while SDS solution could only wash out up to 31% of the total zinc from the soil under similar experimental conditions. pH played a very important role in zinc removal and at pH 4, both soapnut and SDS removed nearly similar amount of zinc. Analysis of the FT-IR data suggested that saponin did not interact chemically with zinc, offering an option for reusing the surfactant after precipitating the zinc by using NaOH at pH of 10.3. Damage to the soil was found to be negligible. This study concludes that soapnut can be used as a washing agent for removal of Zn from high iron soil with minimal damage to the soil.
Graphical Image: Biodegradable plant-based surfactant.